The Element Silver
A comprehensive guide to silver, from its atomic structure to fundamental physical properties to basic history. Silver is a soft, white, lustrous transistion metal that has the highest electrical conductivity and highest thermal conductivity of any metal. The existence of silver has been known since ancient times, and is one of the few metals many recognize by name in more than one language.
|
|
|
|
|
|
|
|
|
|
Periodic Table Properties
|
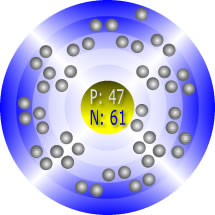
Silver’s Atomic Structure
|
||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
Silver History
The attractive nature of silver is self evident. It has been used for thousands of years for many, many things. Interestingly, silver has some traits that standout above all other metals. Did you know silver has?
- The whitest color
- The highest optical reflectivity
- The highest thermal conductivity
- The highest electrical conductivity
Generally speaking, the majority of today’s mining operations that produce silver are after much more. Silver occurs with other base metals, like gold, copper, zinc and lead. It’s these metals that are typically the thrust of many mines. Silver is just another benefit or byproduct of them.
Obviously, as price adjustments happen with silver the focus or priority of mining it can shift. However, that shifting isn’t as much as one would think. Because silver has properties that make it extremely useful in electronics and photography, a significant portion of its demand is driven by needs rather than price.
In recent history, there have been major events that have resulted in silver price changes:
|
1985 |
U.S. Mint authorized to begin minting a silver bullion coin |
|
1979-80 |
Attempt to corner the silver market |
|
1968 |
Redemption of silver certificates for silver could only be made until June 24; thereafter, silver certificates would be exchanged for Federal Reserve Notes |
|
1967 |
Announcement by U.S. Government that all silver coins would be withdrawn from circulation |
|
1965 |
Silver eliminated from all U.S. coins except the half dollar, which has its silver content reduced from 90% to 40% |
|
1963 |
Silver Purchase Act and various other legislation repealed; U.S. Treasury authorized to print Federal Reserve Notes, which were not redeemable for silver, for circulating currency |
|
1950-68 |
Huge U.S. Government silver holdings largely depleted |
|
(Source of data: U.S. Geological Survey) |
|
Silver Phyiscal Properties & Basic Information
|
Phase: Solid
|
Magnetic Ordering: Diamagnetic
|
|
|
Classification: Transition Metal
|
Electrical Resistivity: (20 °C) 15.87 n Ω·m
|
|
|
Mass: 107.8682
|
Thermal Conductivity: (300 K) 429 W·m−1·K−1
|
|
|
Hardness: 3.25 mohs
|
Thermal Diffusivity: (300 K) 174 mm²/s
|
|
|
Density @ 293 K: 10.49 g/cm3
|
Thermal Expansion: (25 °C) 18.9 µm·m−1·K−1
|
|
|
Atomic Volume: 10.3 cm3/mol
|
Speed of sound (thin rod): (r.t.) 2680 m·s−1
|
|
|
Liquid Density at m.p.: 9.320 g·cm−3
|
Young’s modulus: 83 GPa
|
|
|
Melting Point: 1234.93 K (961.78 °C, 1763.2 °F)
|
Shear modulus: 30 GPa
|
|
|
Boiling Point: 2435 K (2162 °C, 3924 °F)
|
Bulk modulus: 100 GPa
|
|
|
Heat of Fusion: 11.28 kJ·mol−1
|
Poisson ratio: 0.37
|
|
|
Heat of Vaporization: 250.58 kJ·mol−1
|
Mohs hardness: 2.5
|
|
|
Specific Heat Capacity: (25 °C) 25.350 J·mol−1·K−1
|
Vickers hardness: 251 MPa
|
|
|
CAS registry number: 7440-22-4
|
Brinell hardness: 24.5 MPa
|
|
|
|
Radius: ionic radius (2- ion): pm |
Reactions With air: mild, =>Ag2O |
Silver Half Lives: |
|
|
Isotope
|
Half Live
|
|
Ag105
|
41.3 days
|
|
Ag105m
|
7.2 minutes
|
|
Ag106m
|
8.4 days
|
|
Ag107
|
stable
|
|
Ag108
|
2.4 minutes
|
|
Ag108m
|
130 years
|
|
Ag109
|
stable
|
|
Ag109m
|
39.8 seconds
|
|
Ag110
|
24.6 seconds
|
|
Ag110m
|
249.8 days
|
|
Ag111
|
7.47 days
|
![[Most Recent Quotes from www.kitco.com]](http://www.kitconet.com/images/quotes_2a.gif)